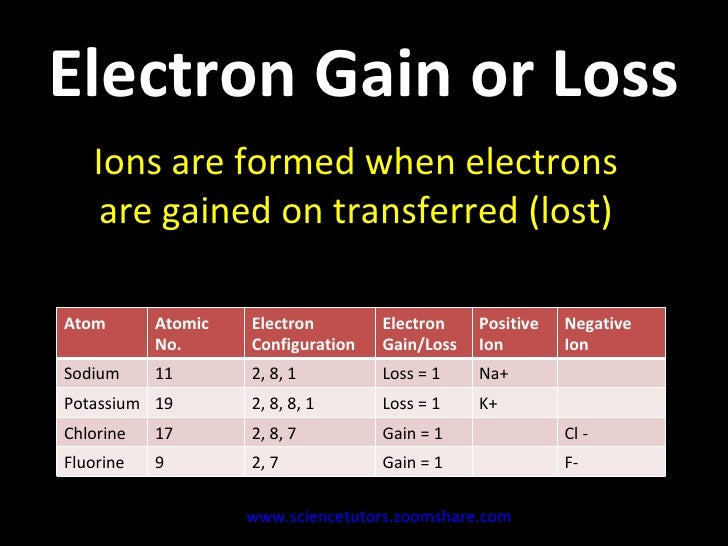
This electron must go into the lowest-energy subshell available, the 3 s orbital, giving a 1 s 2 2 s 2 2 p 6 3 s 1 configuration. Locate the nearest noble gas preceding phosphorus in the periodic table. Then subtract its number of electrons from those in phosphorus to obtain the number of valence electrons in phosphorus. Both nitrogen and phosphorus have 5 valence electrons, but nitrogen has valency of 3 while phosphorus has valency of 3 or 5. Maybe phosphorus can gain 3 electrons or remove 5 electrons.But is it possible to remove 5 electrons? Or is it that phosphorus valence shell is farther away than nitrogen, so it is easier for phosphorus to.
- P Valence Electrons
- Phosphorus Valence Electrons Charge
- Phosphorus Valence Electrons Diagram
- Phosphorus Valence Electrons And Ion
A step-by-step description of how to write the electron configuration for Phosphorus (P). In order to write the P electron configuration we first need to kn.
Lewis structure of phosphate ion is drawn clearly in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of PO43- ion. In lewis structure, there should be charges on atoms.
Phosphate ion | PO43-
Phosphate ion is one of the oxyanion of phosphorous. Phosphorous is at +5 oxidation state in PO43-. Also, phosphate ion has a -3 charge.

Lewis structure of PO43- ion
In the lewis structure of PO43-, three is a double bond between phosphorous atom and one oxygen atom. Between other oxygen atoms, there are only single bonds with phosphorous atom. Also, each oxygen atom has a -1 charge.
Related lewis structures to H3PO4
H3PO2 lewis structureH3PO3 lewis structure
Steps of drawing lewis structure of PO43-
Following steps are required to draw the PO43- lewis structure and they are explained in detail in this tutorial.
- Find total number of electrons of the valance shells of sulfur and oxygen atoms
- Total electrons pairs
- Center atom selection
- Put lone pairs on atoms
- Check the stability and minimize charges on atoms by converting lone pairs to bonds.
Drawing correct lewis structure is important to draw PO43- resonance structures correctly.
Total number of electrons of the valance shells of SO42-
Phosphorous is located at 5th group in the periodic table. Therefore phosphorous has five valence electrons in its last shell. Oxygen atom is located at sixth group in the periodic table and has six valence electrons in its last shell.
- Total valence electrons given by phosphorous atom = 5
There are four oxygen atoms in PO43- ion, Therefore
- Total valence electrons given by oxygen atoms = 6 *4 = 24
There are -3 charge on PO43- ion. Therefore there are three more electrons which comes from outside to contribute to the total valence electrons.
- Total valence electrons = 5 + 24 + 3 = 32
Total valence electrons pairs
Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells
Total electron pairs are determined by dividing the number total valence electrons by two. For, PO43- ion, Total pairs of electrons are 16.
Center atom of PO43- ion
To be the center atom, ability of having greater valance is important. Therefore sulfur has the more chance to be the center atom (See the figure) because sulfur can show valance of 6. Maximum valence of oxygen is two. So, now we can build a sketch of PO43- ion.
Mark electrons as lone pairs on atoms
- There are already four P-O bonds around the phosphorous atom in the above sketch. Therefore only twelve (16-4 = 12) valence electrons pairs are remaining to draw the lewis structure.
- First, mark those twelve valence electrons pairs as lone pairs on outside atoms (on oxygen atoms). One oxygen atom will take three lone pairs following the octal rule (oxygen atom cannot keep more than eight electrons in its valence shell).
- For four oxygen atoms, twelve electrons pairs are spent. Now all electron pairs are spent. There is no electron pairs to mark on phosphorous atom.
Charges on atoms
After, marking electron pairs on atoms, we should mark charges of each atom. Each oxygen atom will get a -1 charge and phosphorous atom get a +1 charge. The overall charge of ion is ( -1*4 + (+1) ) = -3.
Check the stability and minimize charges on atoms by converting lone pairs to bonds
When charges exist everywhere (on atoms) in a ion or a molecule, that structure is not stable. We should try to reduce charges on atoms as much as possible. Now, we are going to learn how to reduce charges of atoms in sulfate ion.
- Oxygen atoms should hold negative charges because electronegativity (3.5) of oxygen is higher than phosphorous (2.1). Otherwise, we can say, ability of holding negative charges is great in oxygen atoms than phosphorous atoms.
- The drawn structure is not a stable one because all atoms have charges.
- Now, we should try to minimize charges by converting lone pair or pairs to bonds. So convert one lone pair of one oxygen atom to make a new P-O bond.
- Now there is a double bond between phosphorous atom and one oxygen atom (one P=O bond). Now, there are three P-O single bonds between phosphorous atom and other three oxygen atoms (three P-O bonds).
You see charges of atoms in PO43- are reduced. Now, there is no charge in one oxygen atom and phosphorous atom. So we have an stable ion than previous one.
Lewis structure of PO43-
Can I reduce charges of atoms furthermore ?
You should know, phosphorous can keep more than eight electrons in its last shell. Therefore we can convert one more lone pair of another oxygen atom to a bond.
But, when you try to do this, phosphorous will get a -1 charge. Earlier in this tutorial, I told you that, most electronegative element should have the negative charges. Therefore, we cannot reduce charges furthermore.
Questions
How many oxygen atoms have charges in PO43- lewis structure?
Three oxygen atoms have charges. One oxygen atom holds - 1 charge and overall there is -3 charge.
In phosphate ion lewis structure, there is -3 charge. Are their charge and lone pairs on phosphorous atom?
There are no any charge on phosphorous atom in phosphate ion lewis structure. Also, there is no lone pair or pairs in phosphate ion lewis structure.
How do I get the lewis structure of H3PO4 from PO43- ?
Three hydrogen atoms are linked to oxygen atoms of PO43- in H3PO4 molecule. There were -1 negative charge on each of oxygen atom and they will be lost due to joining with hydrogen atoms.
Why phosphorous atom will not take a hydrogen atom to make a P-H bond?
If a hydrogen atom is joint to the phosphorous atom, phosphours atom will get a minus charge which is not acceptable because phosphorous has a lower electronegativity than oxygen.
Are there lone pairs on phosphorus atom in phosphate Lewis structure?
Around phosphorus atom, three single bonds and one double bond exist. No lone pairs exist on phosphorus atom. Also, no charge exists on phosphorus atom in Lewis structure of PO43-
Answer: A neutral Phosphorus Atom has five valence electrons. These are contained in the third energy level of the atom.
| The Phosphorus Element as it would be represented on a modern periodic table. Source |
| Bohr Model of a Phosphorus Atom. |
P Valence Electrons
| Electron Configuration of Phosphorus with a Lewis Diagram on the side as well. Source |
| Phosphorus as it moves in a biochemical process. Source |
Phosphorus Valence Electrons Charge
Sources:
Phosphorus Valence Electrons Diagram
http://thechemistryguru.com/phosphorus/Phosphorus Valence Electrons And Ion
